External consultant of the laboratory - Dmitry M Kolpashchikov
Selection of housekeeping genes
Gene-therapeutic agents based on nucleic acids can be used for the treatment of oncological diseases. To treat cancer, we develop our own unique approach using DNA nanotechnology. The therapy strategy is based on suppressing vital housekeeping genes in the presence of other RNA cancer markers. We are faced with the task of finding the most vulnerable gene or set of genes, the silencing of which will lead to cell apoptosis. For this purpose we design antisense oligonucleotides or siRNAs complementary to the genes of interest and estimate the cytotoxicity caused by gene silencing in different cancerous cell lines. The most promising and vulnerable candidates of housekeeping genes we use for the designing of the anticancer gene-therapeutic agents.
Selection of universal cancer markers
We aim to develop safe and selective gene-therapeutic technologies. The vital housekeeping genes cannot be suppressed directly because in this case the multiple side effects will occur in the normal tissues. To reach the therapeutic apoptosis selectively in the cancerous cell, we develop cancer marker- dependent DNA constructs. This type of the gene-therapeutic agent will activate the therapy (gene silencing) only after interaction with the cancer markers. As the cancerous marker we use miRNAs, messenger RNAs with single nucleotide polymorphism and other nucleic acids. The global task is to find the common cancer markers that are overexpressed in the several types of tumors to design the universal gene-therapeutic agents for the cancer treatment.
Development of nucleic acid based DNA-Machines with Oncomarker dependent therapeutic function
We develop smart cancer marker-dependent DNA constructs using the established oligonucleotides technologies that confirmed their efficiency in clinical practice. Our research focuses on the technology of antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and DNAzymes. They demonstrate advantages, among which high productivity, potential to target almost any genes of interest, simplicity of their design, and the variety of their applications. Thus, our task is combining these promising technologies with our advanced approach to targeting housekeeping genes in the cancerous cells.
Our projects include the DNAzymes-based anticancer DNA-machines, Smart logic gates systems for intracellular computation (figure 1), Binary antisense oligonucleotides agents (figure 2), Antisense DNA-thresholders controlled by cancer marker concentration, and shRNA releasing cassettes. All designs go through two stages: in vitro optimization and intracellular testing.
We strive to develop research in the field of oncology and lay the foundation for the development of new drugs for the individual treatment of oncological diseases.
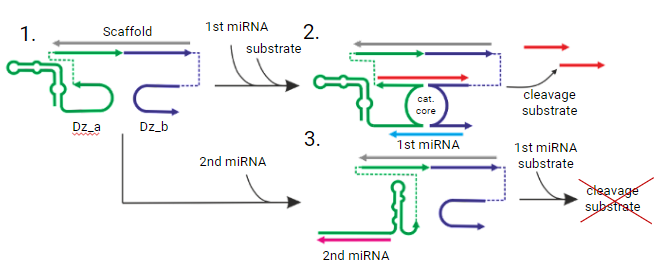
Figure 1. The principle of the logic gates operation activated by a cancer marker (1st miRNA) for cleavage of RNA substrate (red line). The oncosuppressor (2nd miRNA) inhibits the formation of the catalytic core and abolishes the cleavage of the substrate.
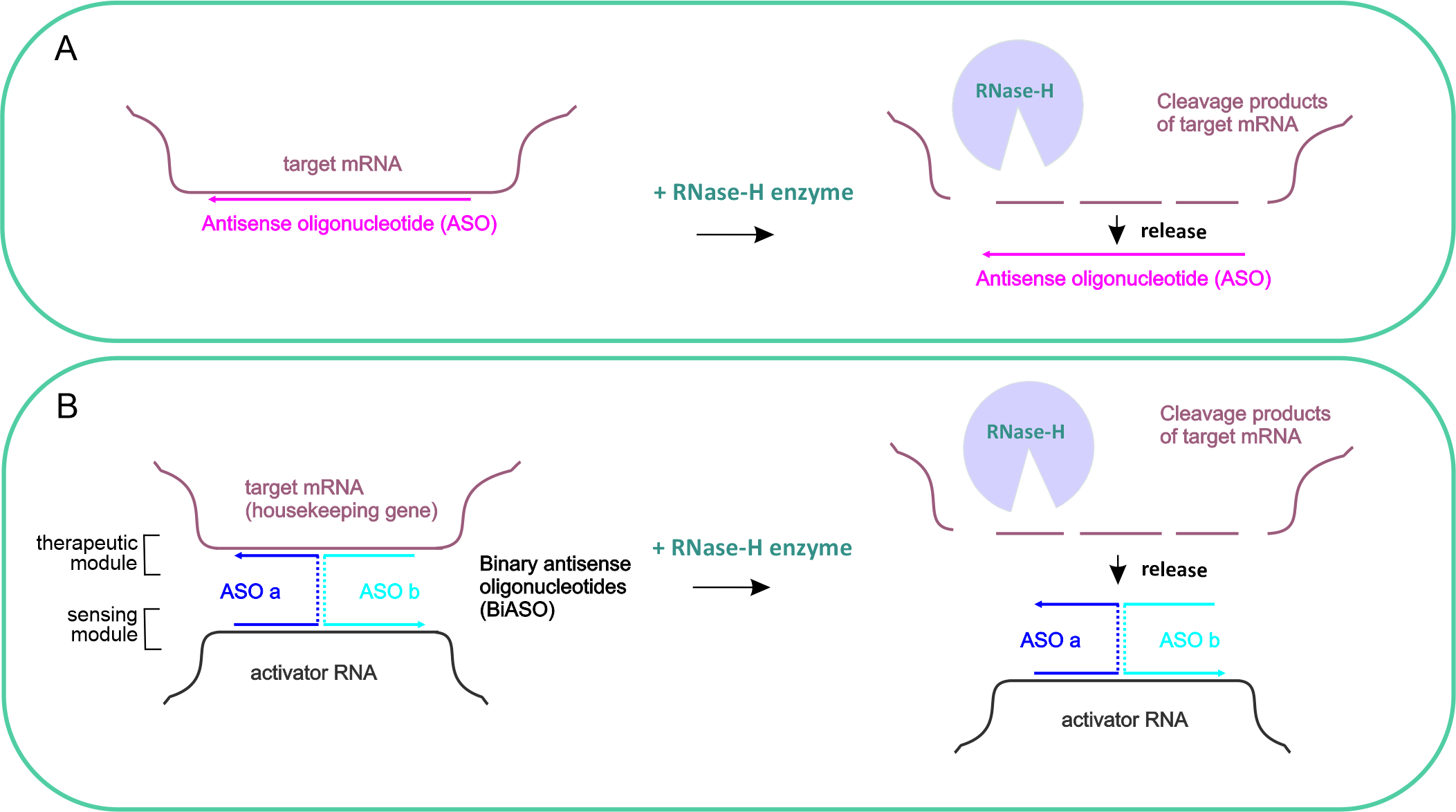
Figure 2. Panel A - the principle of the conventional antisense oligonucleotides. Panel B - the principle of the binary antisense oligonucleotides with therapeutic and sensnsing modules activated in the presence of the activator RNA (cancer marker)
Development of intracellular delivery and oligonucleotide protection research
One of the main limitations of all oligonucleotide-based gene silencing agents in cancer therapy is the insufficient delivery to the targeted cells and the low stability in vivo. We make efforts to develop successful intracellular delivery systems based on rationally designed DNA associations attached to bioconjugates, such as cholesterol moieties (figure 3). To increase the stability of our constructs in the physiological conditions without affecting their efficiency and affinity, we develop a strategy of chemical modification using gamper and mixmer approaches. Moreover, we aim to reduce the in vivo toxic effects of the commonly used chemical modifications by developing smart DNA associations that will act as protection systems for our DNA constructs. By achieving these goals we hope to expand the application of gene-therapeutic agents.
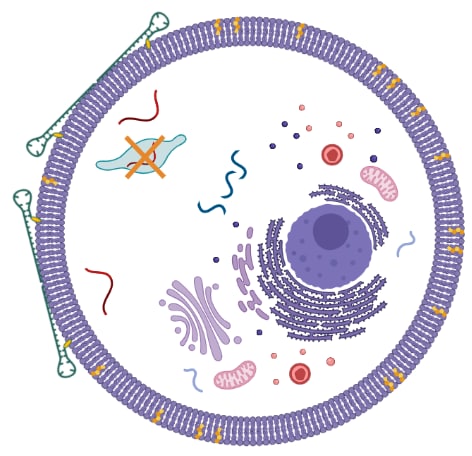
Figure 3. Schematic representation of cholesterol-conjugated DNA associations releasing the ASO agent into the intracellular space