Авторы: Михайлов В.И., Масленникова Т.П., Кривошапкина Е.Ф., Тропников Е.М., Кривошапкин П.В.
Abstract
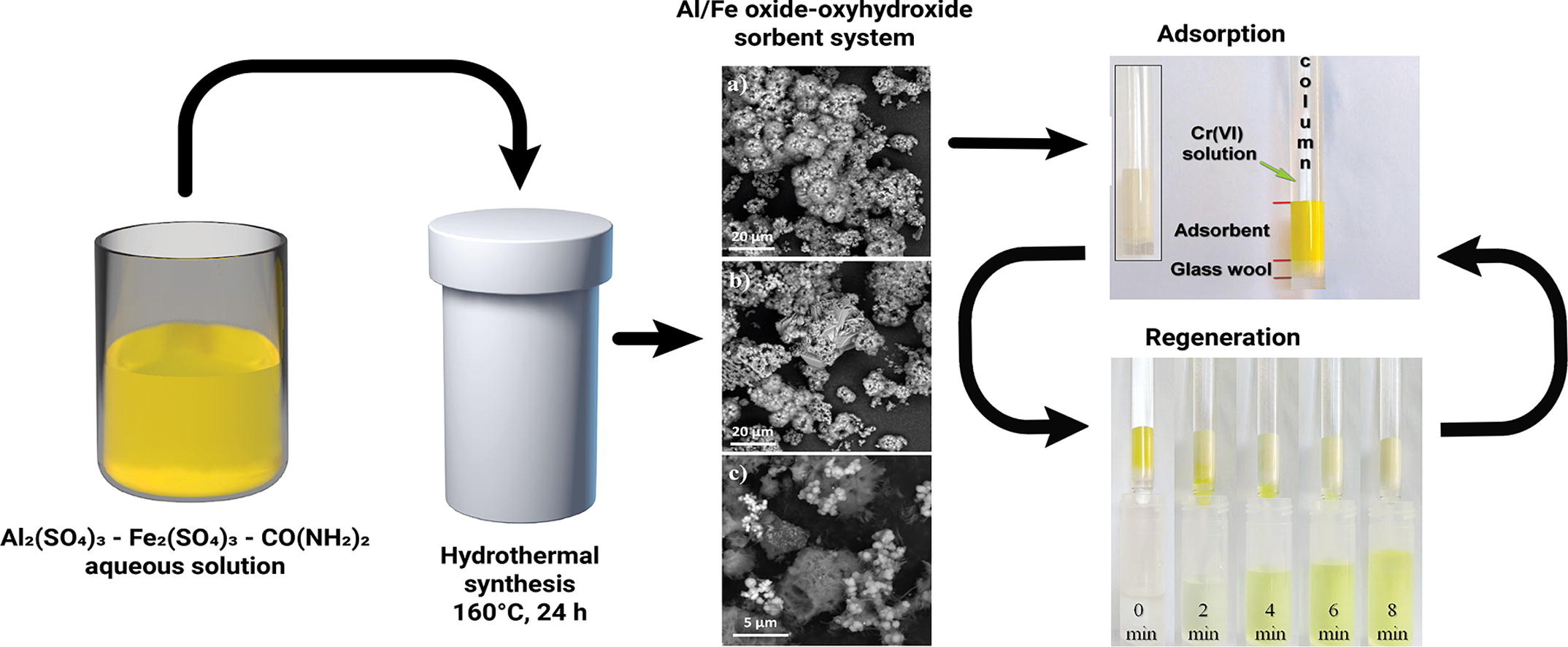
Today, one of the most important global tasks is the treatment of waste water from various pollutants, including heavy metal. Adsorption is a promising method of wastewater treatment in terms of efficiency and cost. In this paper, the Al/Fe oxide-oxyhydroxide composite powders, prepared by hydrothermal method using metal sulfate solutions and urea as precursors, are investigated as adsorbents for chromium. The influence of the [Al3+]:[Fe3+] ratio on the phase composition, textural properties, morphology, and thermal effects of composites was also investigated. It is shown that the maximum specific surface area (190 m2/g) is reached for samples synthesized from solutions with [Al3+]:[Fe3+] = 1:0 and 1:1. Cr(VI) adsorption from solutions was performed under static (batch adsorption) and dynamic (column adsorption) conditions. It is shown that the presence of sulfate ions on the surface of Al-containing samples suppresses the Cr(VI) adsorption. The maximum adsorption capacity in batch adsorption (3.66 mg/g) has a sample synthesized from solutions with [Al3+]:[Fe3+] = 1:6, which is characterized by a high (but not maximal) surface area (102 m2/g), a low content of sulfates and maximum zeta potential. Sorption experiments showed that the mean free adsorption energy is below 8 kJ/mol for all samples, indicating the adsorption process as the physical in nature and suggesting the possibility of Cr(VI) desorption and adsorbent regeneration. Based on the results of fixed-bed adsorption experiments, an atypical form of breakthrough curves is noted, as well as an increase in sorption capacity of about 4–5 times as compared with batch adsorption.
DOI: https://doi.org/10.1016/j.cej.2018.05.023
Read Full:
https://www.sciencedirect.com/science/article/pii/S1385894718308131