Authors: Mikhaylov V.I., Maslennikova T.P., Krivoshapkina E.F., Tropnikov E.M. , Krivoshapkin P.V.
Abstract
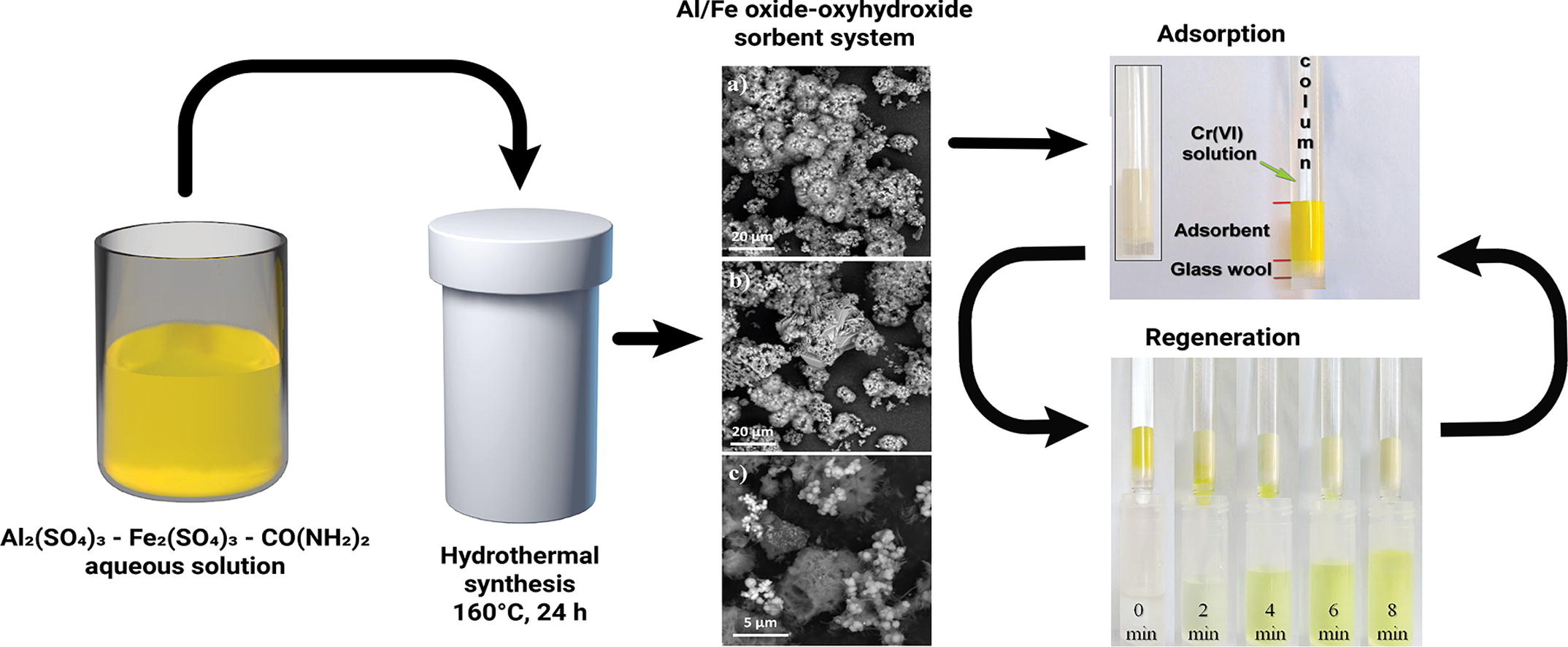
Today, one of the most important global tasks is the treatment of waste water from various pollutants, including heavy metal. Adsorption is a promising method of wastewater treatment in terms of efficiency and cost. In this paper, the Al/Fe oxide-oxyhydroxide composite powders, prepared by hydrothermal method using metal sulfate solutions and urea as precursors, are investigated as adsorbents for chromium. The influence of the [Al3+]:[Fe3+] ratio on the phase composition, textural properties, morphology, and thermal effects of composites was also investigated. It is shown that the maximum specific surface area (190 m2/g) is reached for samples synthesized from solutions with [Al3+]:[Fe3+] = 1:0 and 1:1. Cr(VI) adsorption from solutions was performed under static (batch adsorption) and dynamic (column adsorption) conditions. It is shown that the presence of sulfate ions on the surface of Al-containing samples suppresses the Cr(VI) adsorption. The maximum adsorption capacity in batch adsorption (3.66 mg/g) has a sample synthesized from solutions with [Al3+]:[Fe3+] = 1:6, which is characterized by a high (but not maximal) surface area (102 m2/g), a low content of sulfates and maximum zeta potential. Sorption experiments showed that the mean free adsorption energy is below 8 kJ/mol for all samples, indicating the adsorption process as the physical in nature and suggesting the possibility of Cr(VI) desorption and adsorbent regeneration. Based on the results of fixed-bed adsorption experiments, an atypical form of breakthrough curves is noted, as well as an increase in sorption capacity of about 4–5 times as compared with batch adsorption.
DOI: https://doi.org/10.1016/j.cej.2018.05.023
Read Full:
https://www.sciencedirect.com/science/article/pii/S1385894718308131