Авторы: Chong Liu, Guanna Li, Emiel J.M.Hensen,
Abstract
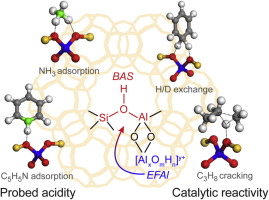
The fundamental aspects of Brønsted acidity and catalytic reactivity of faujasite-type zeolites were investigated by periodic DFT calculations. The adsorption energies of ammonia and pyridine on the Brønsted acid site (BAS) were used to determine the acidity. It is demonstrated that the acid strength of zeolite materials increases with rising Si/Al ratio (low-silica faujasite), and then levels off at high Si/Al ratio (high-silica faujasite). The presence of multinuclear extra framework Al (EFAl) in the sodalite cages substantially enhances the Brønsted acidity. The catalytic reactivity of faujasite toward protolytic propane cracking correlates well with the characterized acidity by base adsorption. However, for H/D exchange reaction of benzene the presence of EFAl species can induce deviations between the measured acidity and the reactivity of faujasite catalysts, indicating that acidity and reactivity are not always directly correlated.
DOI: 10.1016/j.jcat.2016.10.027
Read Full Here:
https://www.sciencedirect.com/science/article/pii/S0021951716302482?via%3Dihub