Авторы: Mikhail Polynski,
Abstract
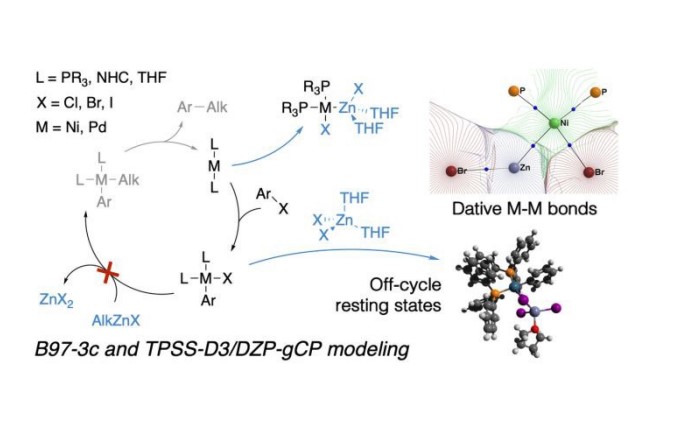
The formation of M-Zn-intermetallic species (M = Ni, Pd) in the course of the Negishi reaction in THF solvent and their potential impact on the in situ catalyst inhibition was investigated by DFT calculations carried out at at two levels of theory. The solvent coordination to the transition metal centers was explicitly accounted for in the calculations. Two model PR3 ligands (PR3 = PMe3 and PPh3), as well as a model NHC-type ligand (1,3-diisopropylimidazol-2-ylidene), were selected. The formation of the intermetallic species during the catalytic process effectively removes the key in-cycle cross-coupling intermediates M(0)L2, cis-[L2PhM(II)X], and trans-[L2PhM(II)X] from the main Negishi catalytic cycle. The reaction between the ZnX2 by-product of the cross-coupling and the formally M(0)L2 species yields bi-metallic adducts with M Zn dative bonds. The nature of these bonds in the resulting complexes was analyzed with the QTAIM. ZnX2 coordination to [L2PhM(II)X] intermediates prevents binding of the alkylzinc halide (RZnX) reagents and blocks the cross-coupling process. Based on these findings, we consider LiX additives as an agent that passivates the Lewis-acidic ZnX2 by-product and limits therefore the product inhibition. This work highlights the profound effect of a system view on catalytic cross-coupling reactions, where interactions between components of catalytic systems are taken into account explicitly and discourage the use of single-cycle-only models.
DOI: 10.1039/C9CY00752K
Read Full Here:
https://doi.org/10.1039/C9CY00752K