Авторы: , ,,, Кучур О.А., Ivan S. Mukhin, Evamarie Hey-Hawkins, Виноградов А.В., Морозов М.И.
Abstract
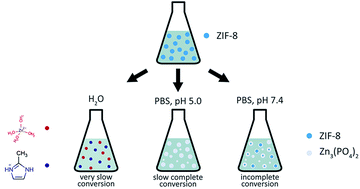
The zeolitic imidazolate framework ZIF-8 (Zn(mim)2, mim = 2-methylimidazolate) has recently been proposed as a drug delivery platform for anticancer therapy based on its capability of decomposing in acidic media. The concept presumes a targeted release of encapsulated drug molecules in the vicinity of tumor tissues that typically produce secretions with elevated acidity. Due to challenges of in vivo and in vitro examination, many studies have addressed the kinetics of ZIF-8 decomposition and subsequent drug release in phosphate buffered saline (PBS) with adjusted acidity. However, the presence of hydrogen phosphate anions [HPO4]2− in PBS may also affect the stability of ZIF-8. As yet, no separate analysis has been performed comparing the dissolving capabilities of PBS and various acidification agents used for regulating pH. Here, we provide a systematic study addressing the effects of phosphate anions with and without lactic acid on the degradation rate of ZIF-8 microcrystals. Lactic acid has been chosen as an experimental acidification agent, since it is particularly secreted by tumor cells. Interestingly, the effect of a lactic acid solution with pH 5.0 on ZIF-8 degradation is shown to be weaker compared to a PBS solution with pH 7.4. However, as an additive, lactic acid is able to enhance the decomposition efficacy of other solutions by 10 to 40 percent at the initial stage, depending on the presence of other ions. Additionally, we report mild toxicity of ZIF-8 and its decomposition products, as examined on HDF and A549 cell lines.
Read full: https://pubs.rsc.org/en/content/articlelanding/2021/RA/D1RA07089D