Authors: Roderigh Y. Rohling, Emiel J. M. Hensen,
Abstract
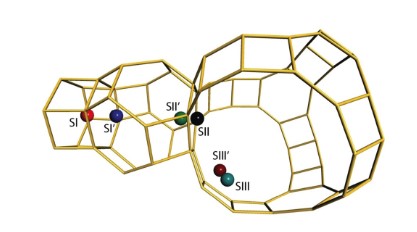
The catalytic Diels–Alder cycloaddition–dehydration (DACD) reaction of furanics with ethylene is a promising route to bio‐derived aromatics. The reaction can be catalyzed by alkali‐metal‐exchanged faujasites. Herein, the results of periodic DFT calculations based on accurate structural models of alkali‐metal‐exchanged zeolites are presented, revealing the fundamental roles that confinement and the nature of the exchangeable cations in zeolite micropores have in the performance of faujasite‐based catalysts in the DACD reaction. Special attention is devoted to analyzing the effect of functional substituents on furanic substrates (furan, 2,5‐dimethylfuran, 2,5‐furandicarboxylic acid) on the catalyst behavior. It is demonstrated that the conventional reactivity theories of the Diels–Alder chemistry based on simplistic single‐site Lewis acidity and substituent effects do not apply if catalytic processes in the multiple‐site confined environment of zeolite nanopores are considered. The nature and cooperativity of the interactions between the multiple exchangeable cations and the substrates determine the reaction energetics of the elementary steps involved in the DACD process.
DOI:10.1002/cphc.201701058
Read Full Here:
http://onlinelibrary.wiley.com/doi/10.1002/cphc.201701058/abstract