Authors: Nikolay Kosinov, Alexandra S. G. Wijpkema, Evgeny Uslamin, Roderigh Rohling, Ferdy J. A. G. Coumans, Brahim Mezari, Alexander Parastaev, Artem S. Poryvaev, Matvey V. Fedin,, Emiel J. M. Hensen
Abstract
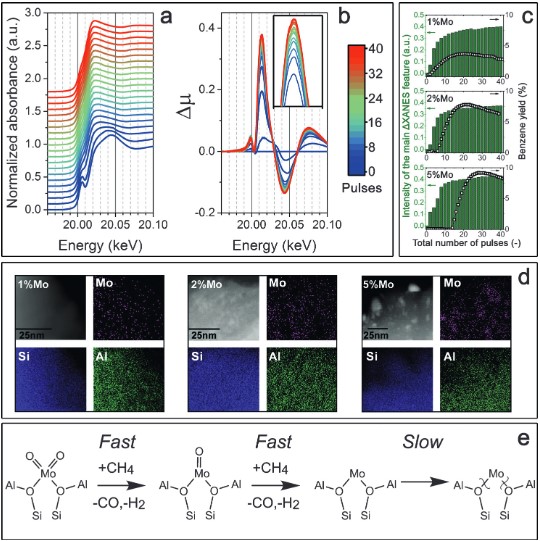
Non‐oxidative dehydroaromatization of methane (MDA) is a promising catalytic process for direct valorization of natural gas to liquid hydrocarbons. The application of this reaction in practical technology is hindered by a lack of understanding about the mechanism and nature of the active sites in benchmark zeolite‐based Mo/ZSM‐5 catalysts, which precludes the solution of problems such as rapid catalyst deactivation. By applying spectroscopy and microscopy, it is shown that the active centers in Mo/ZSM‐5 are partially reduced single‐atom Mo sites stabilized by the zeolite framework. By combining a pulse reaction technique with isotope labeling of methane, MDA is shown to be governed by a hydrocarbon pool mechanism in which benzene is derived from secondary reactions of confined polyaromatic carbon species with the initial products of methane activation.
DOI:10.1002/anie.201711098
Read Full Here:
https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.201711098