Authors: Roderigh Y. Rohling, Evgeny Uslamin, Bart Zijlstra, Ionut C. Tranca, Ivo A. W. Filot, Emiel J. M. Hensen,
Abstract
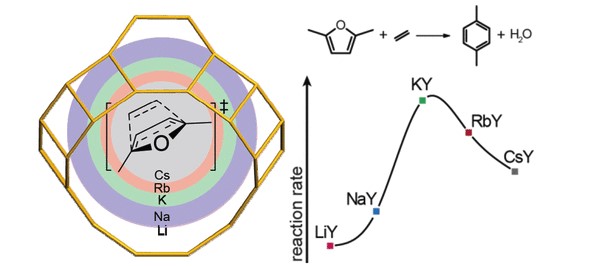
The one-pot Diels–Alder cycloaddition (DAC)/dehydration (D) tandem reaction between 2,5-dimethylfuran and ethylene is a potent pathway toward biomass-derived p-xylene. In this work, we present a cheap and active low-silica potassium-exchanged faujasite (KY, Si/Al = 2.6) catalyst. Catalyst optimization was guided by a computational study of the DAC/D reaction mechanism over different alkali-exchanged faujasites using periodic density functional theory calculations complemented by microkinetic modeling. Two types of faujasite models were compared, i.e., a high-silica alkali-exchanged faujasite model representing isolated active cation sites and a low-silica alkali-exchanged faujasite in which the reaction involves several cations in the proximity. The mechanistic study points to a significant synergetic cooperative effect of the ensemble of cations in the faujasite supercage on the DAC/D reaction. Alignment of the reactants by their interactions with the cationic sites and stabilization of reaction intermediates contribute to the high catalytic performance. Experiments confirmed the prediction that KY is the most active catalyst among low-silica alkali-exchanged faujasites. This work is an example of how the catalytic reactivity of zeolites depends on multiple interactions between the zeolite and reagents.
DOI: 10.1021/acscatal.7b03343
Read full here:
http://pubs.acs.org/doi/abs/10.1021/acscatal.7b03343